CONCISE NOTE ON MOLE CONCEPT, INTEGRATED SCIENCE (SHS)
CONCISE NOTE ON MOLE CONCEPT
Mole
Mole is defined as the amount of substance containing Avogadro’s number in of particles.
A mole is also the number of atoms in exactly 12 grams of carbon-12.
The Avogadro’s number of particles is 6.02 x1023 particles.
A mole is also a unit of measurement of the amount of substance 1 mole of any substance contains 6.0223 particles of that substance.
Mole is also referred to as the amount of substance.
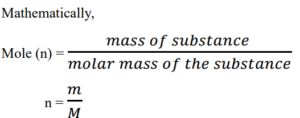
Questions.
1. Helium (He) is a valuable gas used in industry, low-temperature research, deep-sea diving tanks, and balloons. How many moles of Helium are in 6.46 g of Helium? (M = 4g/mol).
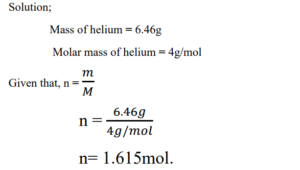
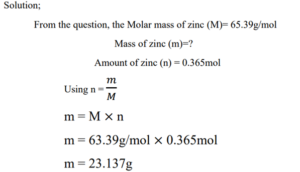
2. Zinc (Zn) is a silvery metal that is used in making brass (with copper) and in plating iron to prevent corrosion. How many grams of Zn are in 0.356 moles of Zn? [M = 65.39g/mol]
3. Methane (CH4) is the principal component of natural gas. How many moles of CH4 are present in 6.07 g of CH4? [C = 12 and H = 1].
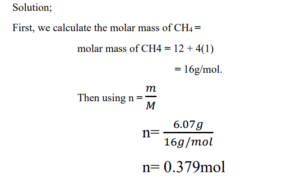
4. Calculate the number of moles of chloroform (CHCl3) in 198 g of chloroform. [C=12, H=1, and Cl=35.5].
5. Calculate the number of moles present in 35.0g of iron metal. [Fe=56g/mol].
6. what is the amount of substance in 9.5g of aluminium. [Al=27g/mol].
Molar mass (Relative molar mass)
Molar mass is the mass of 1 mole of any substance.
It can also be defined as the mass of Avogadro’s number.
The unit for molar mass is g/mol (gram per mole).
Mathematically, the molar mass of a compound can be calculated by adding the atomic masses in g/mol of the constituent atoms.
Relative molar mass is also calculated by adding the relative atomic masses of the constituent atoms.
Questions.
1. Calculate the molar mass of the following compounds.
- Tetraoxosulphate (IV) acid
- CuSO4
- Ammonia
- Sugar
- Sodium chloride
[Relative atomic masses are, H=1.0, C=12, Cu= 63, Cl=35.5, Na=23, N=14, S=32, and O=16].

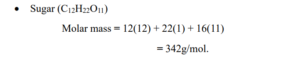
2. What is the molar mass of
- Limestone
- Calcium nitrate
- Methanol
Relationship between Mass (in grams), Molar mass (g/mol), and Amount of substance (in mol).

Avogadro’s constant calculations
It is possible to be asked to find the number of entities/particles in a given mass of substance.
In that situation the formula is;
N = nL where,
N = number of entities/particles in the substance.
L = Avogadro’s number/constant (6.02 x1023).
n = amount of substance in mole
Question.
1. How many molecules of NH3 are present in:
- 5mol of CH4
- 2mol NH3?

2. If you are given 12.04 x1023 molecules of ammonia gas. Calculate the amount of the ammonia molecules. (L = 6.02 x 1023).
Amount of substance concentration [Molar-Mole concentration (Molarity)]
The amount of substance concentration is the concentration of a solution in mol/dm3.
It can also be explained as the amount of substance/solute in mol dissolved in 1dm3 or 1 litre of solution.
The unit of amount of substance concentration is mole per cubic decimetre (mol/dm3).
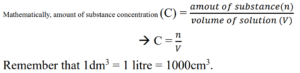
Examples.
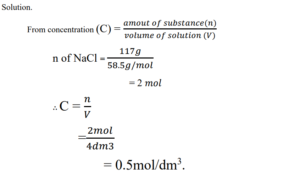
1. 117g of NaCl makes up 4dm3 of salt solution. What is the amount concentration of the solution? (NaCl = 58.5g).
2. What is the concentration in mol/dm3 of 35g of sodium hydroxide dissolved in water to produce 500cm3 of NaOH solution? [R.A.M: H=1, O=16, and Na=23].
3. What is the concentration of a solution in which 41.5g of potassium iodide (KI) is dissolved and made up to 200cm3? (K = 39.0; I = 127.0).
Mass concentration
Mass concentration is the concentration of a solution in grams per cubic decimetre (g/dm3).
It is also explained as the mass of a substance in grams dissolved in 1 decimetre cube (1 dm3) of the mass of a solution.
The unit is gram per mol (g/mol).
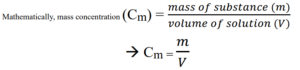
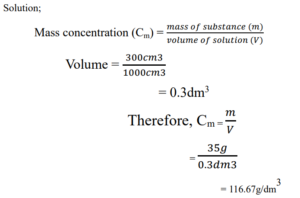
Example:
1. Calculate the mass concentration of 35g of Tetraoxosulphate (VI) dissolved in 300cm3.
2. Calculate the mass of KCl needed to prepare 500cm3 of 0.2M solution.
3. Calculate the concentration of a solution prepared by dissolving 5g of NaCl to make up 500cm3 of solution in;
-
-
- g/dm3
- mol/dm3
-
4. Find the concentration in g/dm3 of 15g of common salt dissolved in water to form a solution of 500cm3.
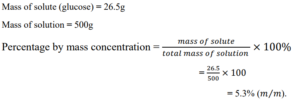
Percentage by mass concentration
It is also known as a weight-by-weight percent.
Percentage by mass concentration is the mass of solute dissolved in the solution divided by the total mass of the solution, multiply by 100 percent
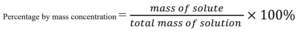
Example:
What is the percentage concentration of a solution containing 26.5g of glucose in 500g of solution?
Percentage by volume concentration
Percentage by volume is explained as the volume of the solute dissolved in the solution divided by the total volume of the solution multiplied by 100 percent.
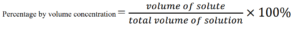
Example,
A solution was prepared by dissolving 25cm3 of glucose in 475cm3 of water. Calculate the concentration of the solution in percentage by volume.

NOTE:
- Atomic masses are measured in atomic mass units (amu), a relative unit based on a value of exactly 12 for the C-12 isotope.
- The atomic mass given for the atoms of a particular element is usually the average of the naturally occurring isotope distribution of that element.
- The molecular mass of a molecule is the sum of the atomic masses of the atoms in the molecule.
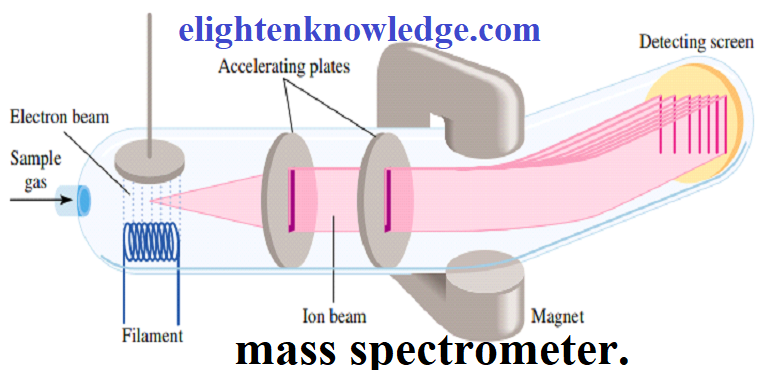
- Both atomic mass and molecular mass can be accurately determined with a mass spectrometer.