Sample preparation to be observed under the light microscope.

Sample preparation to be observed under the light microscope.
Content:
- Dry mount
- Wet mount
- Squash slides
- Smear slides
- Using staining
- Differentiation staining
- Gram staining techniques (gram positive and gram negative)
- Acid-fast technique
- General stages involved in the preparation of slides:
- Risk management
There are a number of different ways in which samples and specimens can be prepared for examination by light microscopy.
The method chosen will depend on the nature of the specimen and the resolution that is desired.
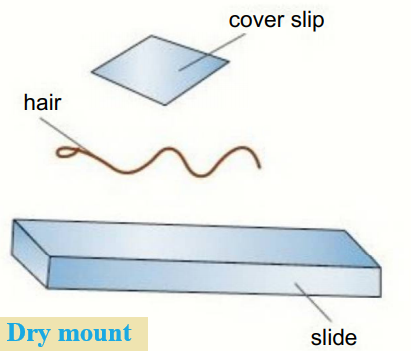
Dry mount:
- Solid specimens are viewed whole or cut into very thin slices with a sharp blade, this is called sectioning.
- The specimen is placed on the centre of the slide and a cover slip is placed over the sample.
- For example, hair, pollen, dust, and insect parts can be viewed whole in this way, and muscle tissue or plants can be sectioned and viewed in this way.
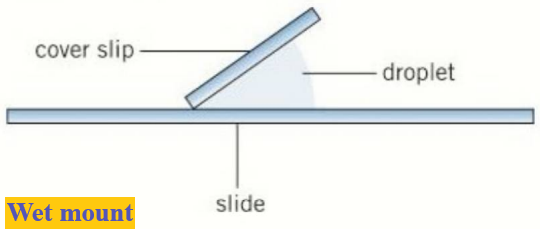
Wet mount:
- Specimens are suspended in a liquid such as water or an immersion oil.
- A cover slip is placed on from an angle.
- For example, aquatic samples and other living organisms can be viewed this way.
Squash slides:
- A wet mount is first prepared, then a lens tissue is used to gently press down the cover slip.
- Depending on the material, potential damage to a cover slip can be avoided by squashing the sample between two microscope slides.
- Using squash slides is a good technique for soft samples.
- Care must be taken to ensure the cover slip is not broken when pressed.
- For example, root tip squashes are used to look at cell division.
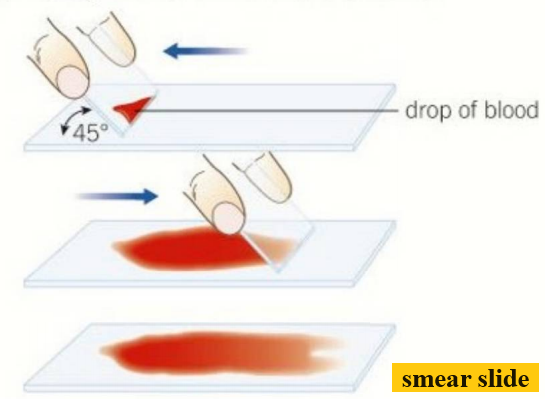
Smear slides:
- The edge of a slide is used to smear the sample, creating a thin, even coating on another slide.
- A cover slip is then placed over the sample.
- An example of a smear slide is a sample of blood.
- This is a good way to view the cells in the blood.
Using Staining:
- In basic light microscopy the sample is illuminated from below with white light and observed from above (brightfield microscopy).
- The whole sample is illuminated at once (wide-field microscopy).
- The images tend to have low contrast as most cells do not absorb a lot of light.
- Resolution is limited by the wavelength of light and diffraction of light as it passes through the sample.
- Diffraction is the bending of light as it passes close to the edge of an object.
- The cytosol (aqueous interior) of cells and other cell structures are often transparent.
- Stains increase contrast as different components within a cell take up stains to different degrees.
- The increase in contrast allows components to become visible so they can be identified.
- To prepare a sample for staining it is first placed on a slide and allowed to air dry.
- This is then heat-fixed by passing through a flame.
- The specimen will adhere to the microscope slide and will then take up stains.
- Crystal violet or methylene blue are positively charged dyes, which are attracted to negatively charged materials in cytoplasm leading to staining of cell components.
- Dyes such as nigrosin or Congo red are negatively charged and are repelled by the negatively charged cytosol.
- These dyes stay outside cells, leaving the cells unstained, which then stand out against the stained background.
- This is a negative stain technique.
Differential staining can distinguish between two types of organisms that would otherwise be hard to identify.
It can also differentiate between different organelles of a single organism within a tissue sample.
Gram stain technique
Gram stain technique is used to separate bacteria into two groups, Gram-positive bacteria and Gram-negative bacteria.
Crystal violet is first applied to a bacterial specimen on a slide, then iodine, which fixes the dye.
The slide is then washed with alcohol.
The Gram-positive bacteria retain the crystal violet stain and will appear blue or purple under a microscope.
Gram negative bacteria have thinner cell walls and therefore lose the stain.
They are then stained with safranin dye, which is called a counterstain.
These bacteria will then appear red.
Gram-positive bacteria are susceptible to the antibiotic penicillin, which inhibits the formation of cell walls.
Gram-negative bacteria have much thinner cell walls that are not susceptible to penicillin.
Acid-fast technique
Acid-fast technique is used to differentiate species of Mycobacterium from other bacteria.
A lipid solvent is used to carry carbolfuchsin dye into the cells being studied.
The cells are then washed with a dilute acid-alcohol solution.
Mycobacterium are not affected by the acid-alcohol and retain the carbolfuchsin stain, which is bright red.
Other bacteria lose the stain and are exposed to a methylene blue stain, which is blue.
General stages involved in the production of slides:
- Fixing – chemicals like formaldehyde are used to preserve specimens in as near-natural a state as possible.
- Sectioning – specimens are dehydrated with alcohols and then placed in a mould with wax or resin to form a hard block. This can then be sliced thinly with a knife called a microtome.
- Staining – specimens are often treated with multiple stains to show different structures.
- Mounting – the specimens are then secured to a microscope slide and a cover slip is placed on top.
Risk management
Many of the stains used in the preparation of slides are toxic or irritants.
A risk assessment must be carried out before any practical is started to identify any procedures involved that may result in harm.
Join Enlighten Knowledge WhatsApp platform.
Join Enlighten Knowledge Telegram platform.