Why you feel pains in the muscle after strenuous exercise? Anaerobic Respiration
Content:
Introduction
Anaerobic respiration in eukaryotic organisms
- Obligate anaerobes:
- Facultative anaerobes
- Obligate aerobes
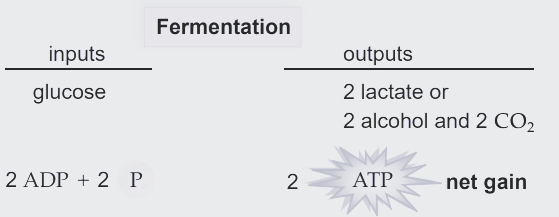
Fermentation
How can food be oxidized without cellular respiration?
Types of fermentation
- Alcohol fermentation
- Lactic acid fermentation
Cause of pains in the muscle after strenuous exercise
Comparing fermentation with aerobic and anaerobic respiration.
Introduction:
Because most of the ATP generated by cellular respiration is due to the work of oxidative phosphorylation, our estimate of ATP yield from aerobic respiration depends on an adequate supply of O2 to the cell.
Without the electronegative oxygen atoms in O2 to pull electrons down the transport chain, oxidative phosphorylation eventually ceases.
However, there are two general mechanisms by which certain cells can oxidize organic fuel and generate ATP without the use of O2:
- anaerobic respiration
- and fermentation.
The distinction between these two is that an electron transport chain is used in anaerobic respiration but not in fermentation.
The electron transport chain is also called the respiratory chain because of its role in both types of cellular respiration.
Anaerobic respiration in eukaryotic organisms
Eukaryotic cells respire aerobically if enough oxygen is available.
Anaerobic respiration, resulting in the synthesis of smaller quantities of ATP, occurs in the absence of oxygen and is also used when oxygen cannot be supplied fast enough to respiring cells.
The use of this less efficient process to produce ATP is a temporary ’emergency’ measure to keep vital processes functioning.
Organisms fall into different categories determined by their dependence on oxygen or not:
Obligate anaerobes:
- Obligate anaerobes cannot survive in the presence of oxygen.
- Almost all obligate anaerobes are prokaryotes,
- Example, Clostridium (bacteria that cause food poisoning), although there are some fungi as well.
Facultative anaerobes
- Facultative anaerobes synthesise ATP by aerobic respiration if oxygen is present, but can switch to anaerobic respiration in the absence of oxygen.
- For example, yeast.
Obligate aerobes
- Obligate aerobes can only synthesise ATP in the presence of oxygen, example, mammals.
- The individual cells of some organisms, such as muscle cells in mammals, can be described as facultative anaerobes because they can supplement ATP supplies by employing anaerobic respiration in addition to aerobic respiration when the oxygen concentration is low.
- However, this is only for short periods and oxygen is eventually required.
- The shortfall of oxygen during the period of anaerobic respiration produces compounds that have to be broken down when oxygen becomes available again, so the organism as a whole is an obligate aerobe.
Fermentation
Fermentation is a way of harvesting chemical energy without using either O2 or any electron transport chain in other words, without cellular respiration.
How can food be oxidized without cellular respiration?
Remember, oxidation simply refers to the loss of electrons to an electron acceptor, so it does not need to involve O2.
Glycolysis oxidizes glucose to two molecules of pyruvate.
The oxidizing agent of glycolysis is NAD+, and neither O2 nor any electron transfer chain is involved.
Overall, glycolysis is exergonic, and some of the energy made available is used to produce 2 ATP (net) by substrate-level phosphorylation.
If O2 is present, then additional ATP is made by oxidative phosphorylation when NADH passes electrons removed from glucose to the electron transport chain.
But glycolysis generates 2 ATP whether oxygen is present or not that is, whether conditions are aerobic or anaerobic.
As an alternative to respiratory oxidation of organic nutrients, fermentation is an extension of glycolysis that allows continuous generation of ATP by the substrate-level phosphorylation of glycolysis.
For this to occur, there must be a sufficient supply of NAD+ to accept electrons during the oxidation step of glycolysis.
Without some mechanism to recycle NAD+ from NADH, glycolysis would soon deplete the cell’s pool of NAD+ by reducing it all to NADH and would shut itself down for lack of an oxidizing agent.
Under aerobic conditions, NAD+ is recycled from NADH by the transfer of electrons to the electron transport chain.
An anaerobic alternative is to transfer electrons from NADH to pyruvate, the end product of glycolysis.
Types of fermentation
Fermentation consists of glycolysis plus reactions that regenerate NAD+ by transferring electrons from NADH to pyruvate or derivatives of pyruvate.
The NAD+ can then be reused to oxidize sugar by glycolysis, which nets two molecules of ATP by substrate-level phosphorylation.
There are many types of fermentation, differing in the end products formed from pyruvate.
Two types are
- alcohol fermentation
- and lactic acid fermentation,
and both are harnessed by humans for food and industrial production.
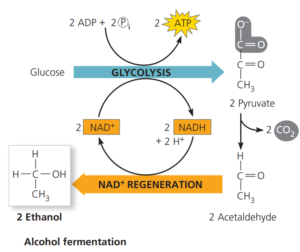
In alcohol fermentation;
pyruvate is converted to ethanol (ethyl alcohol) in two steps.
The first step releases CO2 from the pyruvate, which is converted to the two-carbon compound acetaldehyde.
In the second step, acetaldehyde is reduced by NADH to ethanol.
This regenerates the supply of NAD+ needed for the continuation of glycolysis.
Many bacteria carry out alcohol fermentation under anaerobic conditions.
Yeast (a fungus), in addition to aerobic respiration, also carries out alcohol fermentation.
For thousands of years, humans have used yeast in brewing, winemaking, and baking.
The CO2 bubbles generated by baker’s yeast during alcohol fermentation allow bread to rise.
During lactic acid fermentation;
pyruvate is reduced directly by NADH to form lactate as an end product, regenerating NAD+ with no release of CO2.
Lactate is the ionized form of lactic acid.
Lactic acid fermentation by certain fungi and bacteria is used in the dairy industry to make cheese and yogurt.
A complex series of fermentation and aerobic respiration pathways carried out by yeasts and bacteria on cacao beans is responsible for the production of chocolate.

What about lactate production in humans?
Previously, we thought that human muscle cells only produced lactate when O2 was in short supply, such as during intense exercise.
Research done over the last few decades, though, indicates that the lactate story, in mammals at least, is more complicated.
There are two types of skeletal muscle fibers.
One (red muscle) preferentially oxidizes glucose completely to CO2.
The other (white muscle) produces significant amounts of lactate from the pyruvate made during glycolysis, even under aerobic conditions, offering fast but energetically inefficient ATP production.
The lactate product is then mostly oxidized by red muscle cells in the vicinity, with the remainder exported to liver or kidney cells for glucose formation.
Because this lactate production is not anaerobic, but the result of glycolysis in these cells, exercise physiologists prefer not to use the term fermentation.
Cause of pains in the muscle after strenuous exercise
During strenuous exercise, when carbohydrate catabolism outpaces the supply of O2 from the blood to the muscle, lactate can’t be oxidized to pyruvate.
The lactate that accumulates was once thought to cause muscle fatigue during intense exercise and pain a day or so later.
However, research suggests that, contrary to popular opinion, lactate production actually improves performance during exercise.
Furthermore, within an hour, excess lactate is shuttled to other tissues for oxidation or to the liver and kidneys for production of glucose or its storage molecule, glycogen.
Next day muscle soreness is more likely caused by trauma to cells in small muscle fibers, which leads to inflammation and pain.
Comparing fermentation with aerobic and anaerobic respiration.
Fermentation, anaerobic respiration, and aerobic respiration are three alternative cellular pathways for producing ATP by harvesting the chemical energy of food.
All three-use glycolysis to oxidize glucose and other organic fuels to pyruvate, with a net production of 2 ATP by substrate-level phosphorylation.
And in all three pathways, NAD+ is the oxidizing agent that accepts electrons from food during glycolysis.
Differences
- A key difference is the contrasting mechanisms for oxidizing NADH back to NAD+, which is required to sustain glycolysis.
In fermentation, the final electron acceptor is an organic molecule such as pyruvate (lactic acid fermentation) or acetaldehyde (alcohol fermentation).
In cellular respiration, by contrast, electrons carried by NADH are transferred to an electron transport chain, which regenerates the NAD+ required for glycolysis.
-
Another major difference is the amount of ATP produced.
Fermentation yields two molecules of ATP, produced by substrate-level phosphorylation.
In the absence of an electron transport chain, the energy stored in pyruvate is unavailable.
In cellular respiration, however, pyruvate is completely oxidized in the mitochondrion.
Most of the chemical energy from this process is shuttled by NADH and FADH2 in the form of electrons to the electron transport chain.
There, the electrons move step-wise down a series of redox reactions to a final electron acceptor. (In aerobic respiration, the final electron acceptor is O2; in anaerobic respiration, the final acceptor is another molecule with a high affinity for electrons, although less so than O2.)
Stepwise electron transport drives oxidative phosphorylation, yielding ATP.
Thus, cellular respiration harvests much more energy from each sugar molecule than fermentation can.
-
Obligate anaerobes, carry out only fermentation or anaerobic respiration.
In fact, these organisms cannot survive in the presence of oxygen, some forms of which can actually be toxic if protective systems are not present in the cell.
A few cell types, such as cells of the vertebrate brain, can carry out only aerobic oxidation of pyruvate, and need O2 to survive.
- Other organisms, including yeasts and many bacteria, can make enough ATP to survive using either fermentation or respiration. Such species are called facultative anaerobes.
In yeast cells, for example, pyruvate is a fork in the metabolic road that leads to two alternative catabolic routes.
Under aerobic conditions, pyruvate can be converted to acetyl CoA, and oxidation continues in the citric acid cycle via aerobic respiration.
Under anaerobic conditions, lactic acid fermentation occurs.
Pyruvate is diverted from the citric acid cycle, serving instead as an electron acceptor to recycle NAD+.
To make the same amount of ATP, a facultative anaerobe has to consume sugar at a much faster rate when fermenting than when respiring.