Using Immobilised Enzymes
Enzymes, mainly from microorganisms, have been used indirectly for thousands of years in biotechnologies including brewing, baking, and making cheese and yoghurt.
Many biotechnological processes still use whole microorganisms as their enzyme source.
More recently they are also being used in isolation.
Isolated enzymes
Using isolated enzymes instead of whole organisms has some clear advantages.
- Less wasteful – whole microorganisms use up substrate growing and reproducing, producing biomass rather than product. Isolated enzymes do not.
- More efficient – isolated enzymes work at much higher concentrations than is possible when they are part of the whole microorganism.
- More specific; no unwanted enzymes are present, so no wasteful side reactions take place.
- Maximise efficiency – isolated enzymes can be given ideal conditions for maximum product formation, which may differ from those needed for the growth of the whole microorganism.
- Less downstream processing – pure product is produced by isolated enzymes. Whole microorganisms give a variety of products in the final broth, making the isolation of the desired product more difficult and therefore expensive.
Most of the isolated enzymes used in industrial processes are extracellular enzymes produced by microorganisms.
They are generally easier and therefore cheaper to use than intracellular enzymes.
- Extracellular enzymes are secreted, making them easy to isolate and use.
- Each microorganism produces relatively few extracellular enzymes, making it easy to identify and isolate the required enzyme. In comparison, each microorganism produces hundreds of intracellular enzymes which would need extracting from the cell and separating.
- Extracellular enzymes tend to be much more robust than intracellular enzymes. Conditions outside a cell are less tightly controlled than conditions in the cytoplasm, so extracellular enzymes are adapted to cope with greater variations in temperature and pH than intracellular enzymes.
However, despite the advantages of using extracellular enzymes, intracellular enzymes are still sometimes used as isolated enzymes in manufacturing processes.
This is because there is a bigger range of intracellular enzymes (bullet 2) so in some cases they provide the ideal enzyme for a process.
In these cases, the benefits of using a very specific intracellular enzyme outweigh the disadvantages of the more expensive extraction and isolation process and the need for more lightly controlled conditions.
Examples of intracellular enzymes used as isolated enzymes in industry include;
- glucose oxidase for food preservation,
- asparaginase for cancer treatment,
- and penicillin acylase for converting natural penicillin into semi-synthetic drugs which are more effective.
Immobilized enzymes
Isolated enzymes are more efficient than whole organisms, but using free enzymes is often very wasteful.
Enzymes are not cheap to produce, but at the end of the process they cannot usually be recovered and so they are simply lost.
Increasingly enzymes used in industrial processes are immobilised and attached to an inert support system over which the substrate passes and is convened to product.
This is a case of technology mimicking nature enzymes in cells are usually bound to membranes to carry out their repeated cycles of catalysis.
Because immobilized enzymes are held stationary during the catalytic process, they can be recovered from the reaction mixture and reused time after time.
The enzymes do not contaminate the end product, so less downstream processing is needed.
These things all make the process more economical.
Advantages of using immobilised enzymes
- Immobilised enzymes can be reused which is cheaper.
- Easily separated front the reactants and products of the reaction they are catalysing so reduced downstream processing which is cheaper.
- More reliable there is a high degree of control over the process as the insoluble support provides a stable microenvironment for the immobilised enzymes.
- Greater temperature tolerance immobilised enzymes are less easily denatured by heat and work at optimum levels over a much wider range of temperatures, making the bioreactor less expensive to run.
- Ease of manipulation the catalytic properties of immobilised enzymes can be altered to fit a particular process more easily than those of free enzymes
For example, immobilised glucose isomerase can be used continuously for over 1000 hours at temperatures of 60-65°C.
The ability to keep bioreactors running continuously for long periods without emptying and cleaning helps to keep running costs low.
Disadvantages of using immobilised enzymes
- Reduced efficiency the process of immobilising an enzyme may reduce its activity rate.
- Higher initial costs of materials immobilised enzymes are more expensive than free enzymes or microorganisms. However, the immobilised enzymes, unlike free enzymes, do not need to be replaced frequently.
- Higher initial costs of bioreactor the system needed to use immobilised enzymes is different from traditional fermenters so there is an initial investment cost.
- More technical issues reactors which use immobilised enzymes are more complex than simple fermenters they have more things which can go wrong.
Using immobilised enzymes
Immobilised enzymes are very useful when large quantities of product are wanted, because they allow continuous production. Examples include:
- Immobilised penicillin acylase used to make semi-synthetic penicillin’s from naturally produced penicillin. Many types of bacteria have developed resistance to naturally occurring penicillin so they are no longer very effective drugs. Fortunately, many bacteria are still vulnerable to the semi-synthetic penicillin produced by penicillin acylase so they are very important in treating infections caused by bacteria resistant to the original penicillin. Hundreds of tonnes of these medicines are made every year by immobilised penicillin acylase.
- Immobilised glucose isomerase used to produce fructose from glucose. Fructose is much sweeter than sucrose or glucose and is widely used as a sweetener in the food industries. Glucose is produced from cheap, starch-rich plant material. Glucose isomerise is then used to turn the cheap glucose into very marketable fructose.
- Immobilised lactase used to produce lactose-free milk. Some people, and cats, are intolerant of lactose (milk sugar). Immobilised lactase hydrolyses lactose io glucose and galactose, giving lactose- free milk.
- Immobilised amino acylase used to produce pure samples of L-amino acids used in the production of pharmaceuticals, organic chemicals, cosmetics, and food.
- Immobilised glucoamylase, which can be used to complete the breakdown of starch to glucose syrup. Amylase enzymes break starch down into short chain polymers called dextrin’s. The final breakdown of dextrin’s to glucose is catalysed by immobilised glucoamylase.
- Immobilised nitrile hydratase, an enzyme which is playing an increasing role in the plastics industry. Acrylamide is a very important compound which is used in the production of many plastics. It is made by the hydration of acrylonitrile. Traditionally the hydration of acrylonitrile to acrylamide was done using sulphuric acid with a reduced copper catalyst, but the conditions needed are extreme and therefore expensive. Furthermore, unwanted by-products form and the yield is poor. Using immobilised nitrile hydratase, the conversion takes place under moderate conditions so the process is cheaper and it also gives a 99% yield and no unwanted by-products.
Immobilised enzymes in medicine
Immobilised enzymes and microbial cells are increasingly important both as diagnostic tools in medicine – the manufacture of drugs – for example, the fungus Rhizopus orrhizus is immobilised and used in the production of the steroid drug cortisone.
Biosensors are used in accurate monitoring of blood and urine levels of substances such as glucose, urea, amino acids, ethanol, and lactic acid, as well as in the monitoring of waste treatment, water analysis, and the control of complex chemical processes. They are based on an electrochemical sensor in close proximity to an immobilised enzyme membrane. The enzymes react with a specific substrate and the chemicals produced are detected by the sensor. Because the size of the response is related to the concentration of the substrate, these sensors can be very sensitive and accurate in their measurements and so can be used, for example, by people with diabetes to determine their blood glucose levels and therefore judge the insulin dose they need.
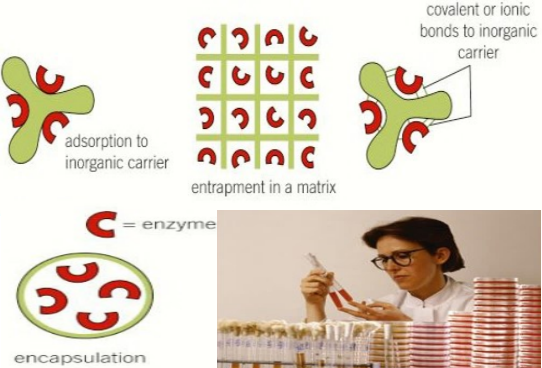
Method of immobilising the enzymes, advantages, and disadvantages
- surface immobilisation – covalent or ionic bonding to inorganic carriers
- covalent bonding, e.g., carriers with amino, hydroxyl, carboxyl groups
- ionic bonding, e.g., polysaccharides such as cellulose, synthetic polymers
Advantages;
- cost varies
- enzymes strongly bound and therefore unlikely to be lost
- enzymes very accessible to substrate pH and substrate concentration often
- have little effect on enzyme activity
Disadvantages
- cost varies
- active site of the enzyme may be modified in process, making it less effective
- surface immobilisation – adsorption to inorganic carriers, e.g., cellulose, silica, carbon nanotubes, and polyacrylamide gel
Advantages;
- simple and cheap to do
- can be used with many different processes
- enzymes very accessible to substrate and their activity is virtually unchanged
Disadvantage;
- enzymes can be lost from matrix relatively easily
- entrapment – in matrix, e.g., polysaccharides, gelatin, activated carbon
Advantage;
- widely applicable to different processes
Disadvantages;
- may be expensive
- can be difficult to entrap
- diffusion of the substrate to and product from the active site can be slow and hold up the reaction
- effect of entrapment on enzyme activity very variable, depending on matrix
- entrapment – membrane entrapment in microcapsules (encapsulation] or behind a semi-permeable membrane, e.g., polymer-based semi-permeable membranes
Advantages;
- relatively simple to do
- relatively small effect on enzyme activity
- widely applicable to different processes
Disadvantages;
- relatively expensive
- diffusion of the substrate to and product from the active site can be slow and hold up the reaction
NOTE:
In some cases, whole microorganisms rather than just the enzymes are immobilised. This has many of the same advantages but avoids the time-consuming and expensive process of extracting the pure enzyme and immobilising it before the process starts. On the other hand, the organisms need food, oxygen, and a carefully controlled environment to work at their optimum rate.