Understanding Cloning in Animals
Cloning is a natural part of the reproductive cycle in many plants.
Perhaps surprisingly, it is not uncommon in many animal species and even occurs in human beings.
Natural cloning in animal
Natural cloning is common in invertebrate animals.
Although it is less common in vertebrates, it still occurs in the form of twinning.
Cloning in invertebrates
Natural cloning in invertebrates can take several forms.
Some animals, such as starfish, can regenerate entire animals from fragments of the original if they are damaged.
Flatworms and sponges’ fragment and form new identical animals as part of their normal reproductive process, all clones of the original.
Hydra produces small ‘buds’ on the side of their body which develop into genetically identical clones.
In some insects, females can produce offspring without mating.
Scientists are increasingly finding differences between the mother and daughters, however, suggesting that as a result of high mutation rates the offspring are not true clones.
Cloning in vertebrates
The main form of vertebrate cloning is the formation of monozygotic twins (identical twins).
The early embryo splits to form two separate embryos.
No one is sure of the trigger which causes this to happen.
The frequency at which identical twins occur varies between species.
For example, domestic cattle rarely if ever produce identical twins naturally, while the incidence in natural human pregnancies is around 3 per 1000.
When monozygotic twins are born, although genetically identical, they may look different as a result of differences in their position and nutrition in the uterus.
Some female amphibians and reptiles will produce offspring when no male is available.
The offspring are often male rather than female, so they are not clones of their mother, yet all of their genetic material arises from her.
Artificial clones in animals
It is relatively easy to produce artificial clones of some invertebrates liquidise a sponge or chop up a starfish and new animals will regenerate from most of the fragments.
It is much more difficult to produce artificial clones of vertebrates, especially mammals.
However, two methods are now used widely in the production of high-quality farm animals and in the development of genetically engineered animals for pharming.
Artificial twinning
After an egg is fertilised, it divides to form a ball of cells. Each of these individual cells is totipotent; it has the potential to form an entirely new animal.
As the cells continue to divide, the embryo becomes a hollow ball of cells.
Soon after this, the embryo can no longer divide successfully.
In natural twinning, an early embryo splits and two foetuses go on to develop from the two halves of the divided embryo.
In artificial twinning, the same thing happens, but the split in the early embryo is produced manually.
The early embryo may be split into more than two pieces and resulting in a number of identical offspring.
Artificial twinning, like embryo transfer which preceded it, is used by the farming community to produce the maximum offspring from particularly good dairy or beef cattle or sheep.

The stages of artificial twinning in cattle can be summarised as follows:
- A cow with desirable traits is treated with hormones so she super ovulates, releasing more mature ova than normal.
- The ova may be fertilised naturally, or by artificial insemination, by a bull with particularly good traits. The early embryos are gently flushed out of the uterus.
- Alternatively, the mature eggs are removed and fertilised by top quality bull semen in the lab.
- Usually before or around day six, when the cells are still totipotent, the cells of the early embryo are split to produce several smaller embryos, each capable of growing on to form a healthy full-term calf.
- Each of the split embryos is grown in the lab for a few days to ensure all is well before it is implanted into a surrogate mother. Each embryo is implanted into a different mother as single pregnancies carry fewer risks than twin pregnancies.
- The embryos develop into foetuses and are born normally, so a number of identical cloned animals are produced by different mothers.
In pigs, a number of cloned embryos must be introduced into each mother pig.
This is because they naturally produce a litter of piglets, and the body may reject and reabsorb a single foetus.
This technology makes it possible to greatly increase the number of offspring produced by the animals with the best genetic stock.
Some of the embryos may be frozen.
This allows the success of a particular animal to be assessed and, if the stock is good, remaining identical embryos can be implanted and brought to term.
Somatic cell nuclear transfer
Artificial twinning clones an embryo.
However, it is now possible to clone an adult animal, by taking the nucleus from an adult somatic (body) cell and transferring it to an enucleated egg cell (an oocyte which has had the nucleus removed).
A tiny electric shock is used to fuse the egg and nucleus, stimulate the combined cell to divide, and form an embryo that is a done of the original adult.
This process is known as somatic cell nuclear transfer (SCNT).
The first adult mammal to be cloned in this way was Dolly the sheep in 1996.
Since then, scientists have cloned a wide range of species including mice, cows, horses, rabbits, cats, and dogs.
SCNT is simple in theory, although in practice there are many difficulties so the technique is still not widely used.
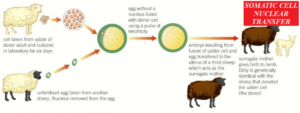
The stages involved are;
- The nucleus is removed from the somatic cell of an adult animal.
- The nucleus is removed from a mature ovum harvested from a different female animal of the same species (it is enucleated).
- The nucleus from the adult somatic cell is placed into the enucleated ovum and given a mild electric shock so it fuses and begins to divide. In some cases, the nucleus from the adult cell is not removed – it is simply placed next to the enucleated ovum and the two cells fuse (electrofusion) and begin to divide under the influence of the electric current.
- The embryo that develops is transferred into the uterus of a third animal, where it develops to term.
- The new animal is a clone of the animal from which the original somatic cell is derived, although the mitochondrial DNA will come from the egg cell.
This process is also known as reproductive cloning because live animals are the end result.
The cloned embryo can then be split to produce several identical clones.
There have been some problems with the animals produced by SCNT.
Dolly the sheep had to be put down when she was only six years old because she suffered from arthritis and lung disease, usually seen in much older sheep.
However, scientists have improved the technique and whilst concerns about premature ageing in clones produced by SCNT persist, researchers in Japan have produced 581 clones from one original donor mouse, through 25 generations.
The mice in each generation were cloned to produce the next generation.
Furthermore, they all had babies naturally to prove they functioned normally.
All of the mice had normal lifespans.
The same team has also produced SCNT clones from the bodies of mice which had been frozen for 16 years.
SCNT can be used in a number of ways.
It is used in pharming the production of animals which have been genetically engineered to produce therapeutic human proteins in their milk.
It can also be used to produce genetically modified (GM) animals which grow organs that have the potential to be used in human transplants.
Pros and cons of animal cloning
Animal cloning is still not widespread, although it is increasingly used in agriculture and the world of animal breeding and medicine.
A number of arguments are put forward both for and against the process.
Arguments for animal cloning
- Artificial twinning enables high-yielding farm animals to produce many more offspring than normal reproduction.
- Artificial twinning enables the success of a sire (the male animal) at passing on desirable genes to be determined.
- If the first cloned embryo results in a successful breeding animal, more identical animals can be reared from the remaining frozen clones.
- The use of meat from animals born to a cloned parent is now permitted in the US.
- SCNT enables GM embryos to be replicated and to develop, giving many embryos from one engineering procedure.
- It is an important process in pharming the production of therapeutic human proteins in the milk of genetically engineered farm animals, such as sheep and goats.
- SCNT enables scientists to clone specific animals, for example, replacing specific pets or cloning top-class race horses. Pet cats and dogs have been cloned in the US at great expense.
- SCNT has the potential to enable rare, endangered, or even extinct animals to be reproduced.
- In theory, the nucleus from dried or frozen tissue could be transferred to the egg of a similar living species and used to produce clones of species that have been dead for a long time.
Arguments against animal cloning
SCNT is a very inefficient process in most animals it takes many eggs to produce a single cloned offspring.
Many cloned animal embryos fail to develop and miscarry or produce malformed offspring.
Many animals produced by cloning have shortened lifespans, although cloned mice have now been developed which live a normal two years.
SCNT has been relatively unsuccessful so far in increasing the populations of rare organisms or allowing extinct species to be brought back to life.
For example, scientists have attempted to clone the gaur and the banteng both extremely rare breeds of wild cattle.
One gaur calf was born in 2001 and died within a couple of days.
Two banteng calves were born in 2003 one was deformed and euthanised, and the other grew normally but its natural lifespan was halved.
The idea of restoring extinct organisms is exciting but scientists are increasingly unconvinced that it will be possible by this method.
Cloning humans
- Scientists have reproduced clones of primates by artificial twinning but it is proving very difficult to produce a SCNT clone of a primate.
- Part of the problem seems to be that the spindle proteins needed for cell division in primate cells are sited very close to the nucleus, so the removal of the nucleus to produce the enucleated primate ovum also destroys the mechanism by which the cell divides. This is not a problem in the ova of many other mammals because the spindle proteins are more dispersed in the cytoplasm.
- In addition, the synchronisation of the stage of the embryo and the state of the reproductive organs of the mother have to be exactly attuned in primates – there seems to be more flexibility in some other mammals.
- In recent years scientists have finally produced embryonic primate stem cell lines by SCNT. This means it may eventually be possible to develop these potentially important therapeutic cells from human beings.
- In most countries there is strict legislation to prevent reproductive cloning of human beings, even if the technical problems of primate cloning are overcome.
A modified version of SCNT has the potential, however, to produce human embryonic stem cells from an adult which could produce cells to be used to grow new tissues for that individual patient.
Research in this process is strictly controlled so it cannot be used for reproductive cloning it is known as therapeutic cloning to make it clear that the end result is not to reproduce a person.
However, this form of SCNT can potentially make it possible to grow replacement organs which will not trigger an immune response in a patient and which will enable us to cure many currently life-threatening conditions.