The Science and Art of Making Vaccines

The Science and Art of Making Vaccines
Why Vaccination?
The goal of vaccination is to trigger an immune response more rapidly and with less harm than a natural infection.
Following an initial encounter with a pathogen, memory immune cells are established; re-exposure to the same pathogen reawakens these memory cells to control the infection and prevent disease.
Smallpox virus, which caused infections that killed, crippled, or disfigured more than 1 in 20 of all humans who ever lived, is the only human virus to be eradicated.
Viral candidates for eradication must possess two essential features:
- the infectious cycle must take place in a single host,
- and infection (or vaccination) must induce life-long immunity.
Vaccination can be active (the host makes its response to a viral preparation) or passive (components of the immune response are obtained from an appropriate donor or donors and injected directly into the patient).
To be effective, a vaccine must induce protective immunity in a fraction of the population that is sufficient to impede person-to-person transmission, a concept called herd immunity.
Active vaccination can occur by administration of virus particles that have been inactivated or are less pathogenic, or by delivery of individual immunogenic proteins or recombinant DNA vectors that encode them.
Inactivated virus particles or purified proteins often do not induce the same magnitude of response as attenuated preparations, unless mixed with adjuvants that stimulate the early inflammatory response.
Art of Making Vaccines
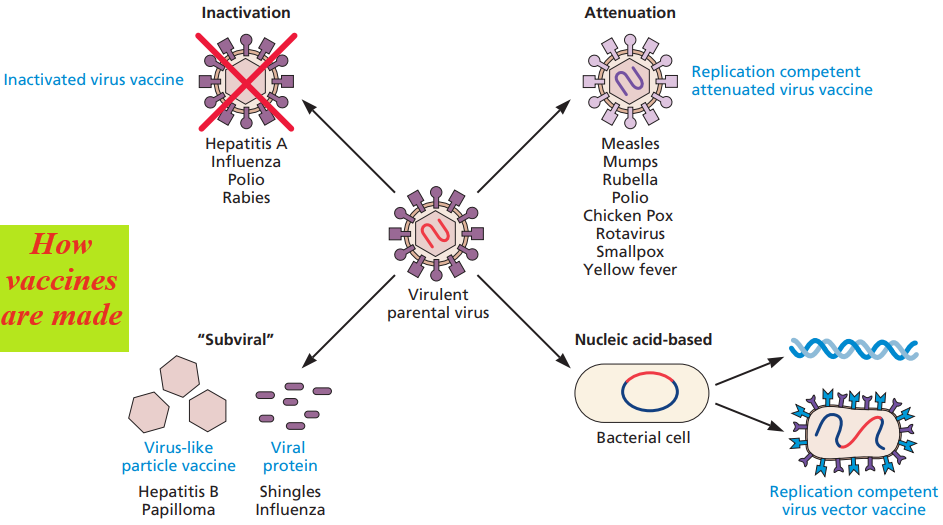
There are four basic approaches to producing vaccines. Each uses components of the pathogenic virus that the vaccine is intended to target.
- A vaccine developer may produce large quantities of the virus of interest and chemically inactivate it (inactivated vaccine),
- attenuate the pathogenicity through laboratory manipulation (replication-competent, attenuated vaccine),
- produce individual proteins free of the viral nucleic acid (subunit vaccine),
- or molecularly clone all or portions of the viral genome for preparation of recombinant nucleic acid vaccines (recombinant vaccine).
Although they are different in formulation, the conceptual underpinnings of all vaccines adhere to principles that would be understood by Pasteur, the father of vaccines.
The most common, commercially successful vaccines simply comprise attenuated or inactivated virus particles.
Inactivated Virus Vaccines
The inactivated poliovirus, influenza virus, hepatitis A virus, and rabies virus vaccines are examples of effective inactivated vaccines administered to humans.
Moreover, inactivated vaccines, such as those that prevent equine influenza virus and porcine circovirus infections, are widely used in veterinary medicine.
To prepare such a vaccine, virulent virus particles are isolated and inactivated by chemical or physical procedures.
These treatments completely eliminate the infectivity of the virus, but not its antigenicity thus, the ability to induce the desired immune response).
Common methods to inactivate virus preparations include treatment with formaldehyde or β-propiolactone, or extraction of enveloped virus particles with non-ionic detergents.
These vaccines are safe for immunodeficient individuals, as the treated viruses cannot reproduce.
Immunization by inactivated vaccines, however, often requires the administration of multiple doses, as the first dose is generally insufficient to produce a protective response.
In principle, inactivated vaccines are very safe, but accidents can and do happen if there is incomplete inactivation of the virulent virus.
Also Read:
- Viral Evolution, Morphology, and Classification
- Adaptive Immunity/Specific Defenses/Acquired Immunity.
- How Pathogens are eliminated from the body and Why the Immune System Reject Foreign Tissues and Organs?
Attenuated Virus Vaccines
Replication-competent, attenuated vaccines are effective for at least two reasons.
Progeny virus particles are produced, but because the virus is severely crippled, reproduction is often restricted to cells around the site of inoculation, resulting in mild or inapparent disease.
However, the limited virus reproduction that does occur stimulates a potent and lasting immune response.
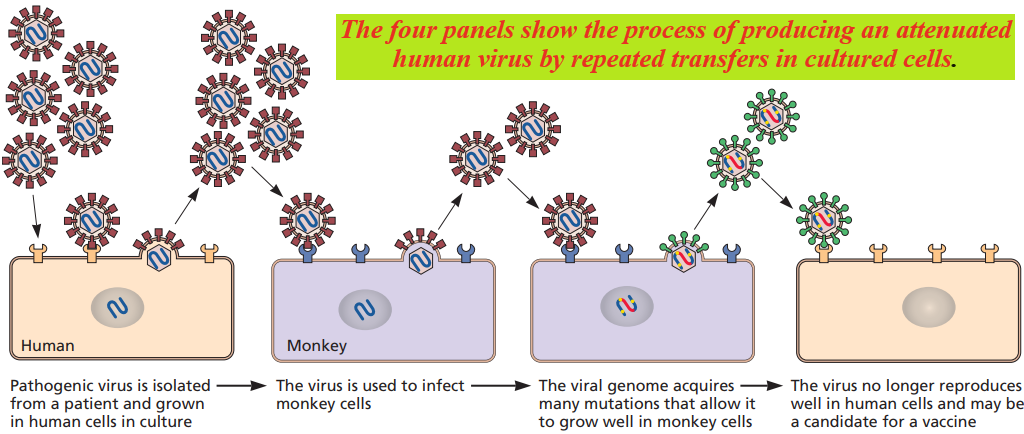
Attenuated viruses are selected by growth in cells other than those of the normal host or by propagation at non-physiological temperatures.
Mutants able to propagate under these conditions are isolated, purified, and subsequently tested for virulence-inappropriate models.
Temperature-sensitive and cold-adapted mutants are often less pathogenic than the parental viruses because of their reduced capacity for reproduction and spread in the warm-blooded host.
In the case of viruses with segmented genomes (e.g., arenaviruses, orthomyxoviruses, bunyaviruses, and reoviruses), attenuated, reassortant viruses may be obtained after mixed infections with pathogenic and non-pathogenic strains.
A good example of attenuation is the oral poliovirus vaccine.
Replication-competent oral poliovirus vaccines in use today comprise three strains selected for attenuated neuro-virulence.
Attenuated vaccines are administered by injection.
Subunit Vaccines
Vaccines formulated with purified components of viruses, rather than the intact particles, are called subunit vaccines.
As only a portion of the viral genome is required for such production, there can be no contamination of the resulting vaccine with the original virus, solving a major safety problem inherent in inactivated virus vaccines.
Determining which viral proteins to include in a subunit vaccine is accomplished by selecting those that are recognized by antibodies and cytotoxic T lymphocytes; this selection can be determined by assessing the immune responses of individuals who have recovered from the disease.
Although the most obvious proteins to choose for subunit vaccines might seem to be those present on the virus surface, this is not uniformly true: nucleocapsid proteins from many RNA viruses, for example, are highly immunogenic.
Importantly, the selected immunogenic viral proteins must be recognized by most individuals for the vaccine to be practical.
This requirement is a major hurdle: as humans are an outbred population, the specificities of immune recognition differ from person to person.
Another approach to developing effective subunit vaccines is to introduce the genes encoding the immunogenic proteins into the genome of non-pathogenic viruses, bacteria, yeasts, insect cells, or plant cells.
Virus-Like Particles
Virus-like particles (VLPs) closely resemble the structure of viruses but are non-infectious because they contain no viral genetic material.
These particles have capsid-like structures that are virtually identical to those in virus particles, but unlike authentic particles, these capsids are empty.
Because the empty capsids retain most of the conformational epitopes seen in the infectious particles (which are often lost in purified protein preparations), virus-like particle vaccines induce durable neutralizing antibodies and other protective responses after injection.
Furthermore, as the particles are completely non-infectious, inactivation with formalin or other agents is not required.
This feature affords at least two additional advantages:
immunogenicity is not compromised (formalin and other alkylating chemicals can alter the conformation of epitopes in inactivated particles),
and concerns about the efficiency of inactivation are avoided.
Virus-like particle vaccines have proven to be particularly attractive for viruses that are propagated poorly in cell culture.
Join Enlighten Knowledge WhatsApp platform.
Join Enlighten Knowledge Telegram platform.
Follow our:
FACEBOOK PAGE.