The fundamentals of quality assurance; everything you need to know.
Quality assurance is what we do to get the right answer for our purpose.
The answer should have sufficient accuracy and precision to support subsequent decisions.
There is no point in spending extra money to obtain a more accurate or more precise answer if it is not necessary.
What are the basics of quality assurance?
Suppose you are cooking for some friends.
While making spaghetti sauce, you taste it, season it, taste it some more.
Each tasting is a sampling event with a quality control test.
You can taste the whole batch because there is only one batch.
Now suppose you run a spaghetti sauce plant that makes 500 pots a day.
You can’t taste each one, so you decide to taste three a day, one each at 9am, 1pm, and 5:30pm.
If the three pots all taste OK, you conclude all 500 are OK.
Unfortunately, that may not be true, but the relative risk that a pot has too much or too little seasoning is not very important because you agree to refund the money of any customer who is dissatisfied.
If the number of refunds is small, say, 60 a year, there is no apparent benefit in tasting four jars a day.
In analytical chemistry, the product is not spaghetti sauce but, rather, raw data, treated data, and results.
Raw data are measurements, such as peak areas from a chromatogram or volumes from a burette.
Treated data are concentrations or amounts found by applying a calibration procedure to the raw data.
Results are what we ultimately report, such as the mean, standard deviation, and confidence interval, after applying statistics to treated data.
Objectives of quality assurance.
A goal of quality assurance is making sure that results meet the customer’s needs.
If you manufacture a drug whose therapeutic dose is just a little less than the lethal dose, you should be more careful than if you make spaghetti sauce.
The kind of data that you collect and the way in which you collect them depend on how you plan to use those data.
A bathroom scale does not have to measure mass to the nearest milligram, but a drug tablet required to contain 2 mg of active ingredient probably cannot contain 2 6 1 mg.
Writing clear, concise use objectives for data and results is a critical step in quality assurance and helps prevent misuse of data and results.
Specifications of quality assurance
Once you have use objectives, you are ready to write specifications stating how good the numbers need to be and what precautions are required in the analytical procedure.
How shall samples be taken and how many are needed?
Are special precautions required to protect samples and ensure that they are not degraded?
Within practical restraints, such as cost, time, and limited amounts of material available for analysis, what level of accuracy and precision will satisfy the use objectives?
What rate of false positives or false negatives is acceptable?
These questions need to be answered in detailed specifications.
Specifications could include required accuracy and precision, reagent purity, apparatus tolerances, the use of certified reference materials, and acceptable values for blanks.
A false positive says that the concentration exceeds the legal limit when, in fact, the concentration is below the limit
A false negative says that the concentration is below the limit when it is actually above the limit.
Even well executed procedures produce some false conclusions because of the statistical nature of sampling and measurement.
Method Validation in Quality Assurance
Where do we start from?
Quality assurance begins with sampling.
We must collect representative samples, and analyte must be preserved after sample is collected.
If our sample is not representative or if analyte is lost after collection, then even the most accurate analysis is meaningless.
Samples for trace metal analysis are usually collected in plastic containers not glass because metal ions found on glass surfaces leach out into the sample over time.
Also, samples for organic analysis are usually collected in glass containers not plastic because organic plasticizers leached from plastic containers can contaminate the sample.
Samples are often stored in the dark in a refrigerator to minimize degradation of organic analytes.
In choosing a method, we also consider
- selectivity
- and sensitivity.
Selectivity means being able to distinguish analyte from other species in the sample (avoiding interference).
Sensitivity is the capability of responding reliably and measurably to changes in analyte concentration.
The detection limit of an analytical method must be lower than the concentrations to be measured.
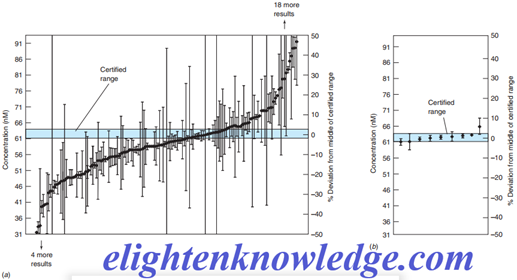
Certified reference materials contain certified levels of analyte in realistic materials that you might be analyzing, such as blood, coal, or metal alloys.
Your analytical method should produce an answer acceptably close to the certified level or there is insufficient accuracy in your method.
-
Blanks account
Blanks account for interference by other species in the sample and for traces of analyte found in reagents used for sample preservation, preparation, and analysis.
Frequent measurements of blanks detect whether analyte from previous samples is carried into subsequent analyses by adhering to vessels or instruments.
-
A method blank
A method blank is a sample containing all components except analyte, and it is taken through all steps of the analytical procedure.
We subtract the response of the method blank from the response of a real sample prior to calculating the quantity of analyte in the sample.
-
A reagent blank
A reagent blank is similar to a method blank, but it has not been subjected to all sample preparation procedures.
The method blank is a more complete estimate of the blank contribution to the analytical response.
-
A field blank
A field blank is similar to a method blank, but it has been exposed to the site of sampling.
A field blank would be a filter carried to the collection site in the same package with the collection filters. The filter for the blank would be taken out of its package in the field and placed in the same kind of sealed container used for collection filters.
The difference between the blank and the collection filters is that air was not sucked through the blank filter.
Volatile organic compounds encountered during transportation or in the field are conceivable contaminants of a field blank.
ALSO;
When dealing with large numbers of samples and replicates, we perform periodic calibration checks to make sure that our instrument continues to work properly and the calibration remains valid.
In a calibration check, we analyze solutions with known concentrations of analyte.
A specification might, for example, call for one calibration check for every 10 samples.
Calibration check solutions should be different from the ones used to prepare the original calibration curve.
This practice helps verify that the initial calibration standards were made properly.
-
Performance test samples
Performance test samples (also called quality control samples or blind samples) are a quality control measure to help eliminate bias introduced by an analyst who knows the concentration of the calibration check sample.
These samples of known composition are provided to the analyst as unknowns.
Results are then compared with the known values, usually by a quality assurance manager.
Together, raw data and results from calibration checks, spike recoveries, quality control samples, and blanks are used to gauge accuracy.
Analytical performance on replicate samples and on replicate portions of the same sample measures precision.
-
Fortification
Fortification also helps ensure that qualitative identification of analyte is correct.
-
Standard operating procedures
Standard operating procedures stating what steps will be taken and how they will be carried out are the bulwark of quality assurance.
For example, if a reagent has “gone bad” for some reason, control experiments built into your normal procedures should detect that something is wrong and your results should not be reported.
It is implicit that everyone follows the standard operating procedures.
Adhering to these procedures’ guards against the normal human desire to take shortcuts based on assumptions that could be false.
How to store/preserve Microorganisms
A meaningful analysis requires a meaningful sample that represents what is to be analyzed.
The sample must be stored in containers and under conditions that do not allow relevant chemical characteristics to change.
Protection might be needed to prevent
- oxidation,
- photodecomposition,
- or growth of organisms.
The chain of custody is the trail followed by a sample from the time it is collected to the time it is analyzed and, possibly, archived.
Documents are signed each time the material changes hands to indicate who is responsible for the sample.
Each person in the chain of custody follows a written procedure telling how the sample is to be handled and stored.
Each person receiving a sample should inspect it to see that it is in the expected condition in an appropriate container.
If the original sample was a homogeneous liquid, but it contains a precipitate when you receive it, the standard operating procedure might dictate that you reject that sample.
Standard operating procedures specify how instruments are to be maintained and calibrated to ensure their reliability.
Many labs have their own standard practices, such as recording temperatures of refrigerators, calibrating balances, conducting routine instrument maintenance, or replacing reagents.
These practices are part of the overall quality management plan.
The rationale behind standard practices is that some equipment is used by many people for different analyses.
We save money by having one program to ensure that the most rigorous needs are met.
Assessment
Assessment is the process of
- collecting data to show that analytical procedures are operating within specified limits
- and verifying that final results meet use objectives.
Documentation is critical for assessment.
Standard protocols provide directions for what must be documented and how the documentation is to be done, including how to record information in notebooks.
For labs that rely on manuals of standard practices, it is imperative that tasks done to comply with the manuals be monitored and recorded. Control charts can be used to monitor performance on blanks, calibration checks, and spiked samples to see if results are stable over time or to compare the work of different employees.
Control charts can also monitor sensitivity or selectivity, especially if a laboratory encounters a wide variety of matrixes.
-
A control chart
A control chart is a visual representation of confidence intervals for a Gaussian distribution.
A control chart warns us when a property being monitored strays dangerously far from an intended target value.
Join the Enlighten Knowledge Community