Method Validation in Quality Assurance
Method validation is the process of proving that an analytical method is acceptable for its intended purpose.
In pharmaceutical chemistry, method validation requirements for regulatory submission include studies of method
- specificity,
- linearity,
- accuracy,
- precision,
- range,
- limit of detection,
- limit of quantitation,
- and robustness.
The fundamentals of quality assurance
Accuracy
Accuracy is “nearness to the truth.” Ways to demonstrate accuracy include
Analyze a certified reference material in a matrix similar to that of your unknown.
Your method should find the certified value for analyte, within the precision of your method.
Compare results from two or more different analytical methods. They should agree within their expected precision.
Analyze a blank sample spiked with a known addition of analyte.
The matrix must be the same as your unknown.
When assaying a major component, three replicate samples at each of three levels ranging from 0.5 to 1.5 times the expected sample concentration are customary.
For impurities, spikes could cover three levels spanning an expected range of concentrations, such as 0.1 to 2 wt.%.
If you cannot prepare a blank with the same matrix as the unknown, then it is appropriate to make standard additions of the analyte to the unknown.
An accurate assay will find the known amount of analyte that was added.
Spiking is the most common method to evaluate accuracy because reference materials are not usually available and a second analytical method may not be readily available.
Spiking with a small volume of concentrated analyte ensures that the matrix remains nearly constant.
Precision
Precision is how well replicate measurements agree with one another, usually expressed as a standard deviation or standard uncertainty (standard deviation of the mean) or confidence interval.
When an experienced analyst replicates his or her measurement by the same procedure using the same instrument, results could be highly repeatable.
The 95% confidence interval could be small.
When different people in different labs with different instruments perform the analysis, each person’s confidence interval could be small, but it might not overlap the confidence intervals of others who did the same analysis.
What are the sources of error?
There could be differences in samples, differences in sample preparation, differences in technique among analysts, uncontrolled changes that occur in each lab from day to day, uncontrolled differences among laboratories, and differences among instruments.
Two broad categories of precision are
- repeatability
- and reproducibility.
Repeatability describes the spread in results when one person uses one procedure to analyze the same sample by the same method multiple times.
Reproducibility describes the spread in results when different people in different labs using different instruments try to follow the same procedure.
Some specific types of precision are defined below:
-
Instrument precision
Instrument precision is the repeatability observed when the same quantity of one sample is repeatedly introduced into an instrument.
Variability could arise from variations in the injected quantity and variations in instrument response.
-
Intra-assay precision
Intra-assay precision is evaluated by analyzing aliquots of a homogeneous material several times by one person on one day with the same equipment.
Each analysis is independent, so the intra-assay precision is telling us how reproducible the analytical method can be.
Intra- assay variability is greater than instrument variability, because more steps are involved. Examples of specifications might be that instrument precision is 1% and intra-assay precision is 2%.
-
Intermediate precision
Intermediate precision, formerly called ruggedness, is the variation observed when an assay is performed by different people on different instruments on different days in the same lab.
Each analysis might incorporate fresh reagents and different chromatography columns.
-
Interlaboratory precision
Interlaboratory precision, which is the same as reproducibility, is the most general measure of reproducibility observed when aliquots of the same sample are analyzed by different people in different laboratories.
Interlaboratory precision can be significantly poorer than intermediate precision.
For example, a 15-laboratory study was conducted to validate a new method for measuring bisphenol A and related phenolic compounds in water.
Intermediate precision (within laboratories) was 1.9 to 5.5% for several compounds.
Interlaboratory precision with all labs following the same instructions was 10.8 to 22.5%.7
Interlaboratory precision becomes poorer as analyte concentration decreases.
-
Linearity
Linearity measures how well a calibration curve follows a straight line, showing that response is proportional to the quantity of analyte.
-
Specificity
Specificity is the ability of an analytical method to distinguish the analyte from everything else that might be in the sample.
Electrophoresis is an analytical method in which substances are separated from one another by their differing rates of migration in a strong electric field.
An electropherogram is a graph of detector response versus time in an electrophoretic separation.
-
Range
Range is the concentration interval over which linearity, accuracy, and precision are all acceptable.
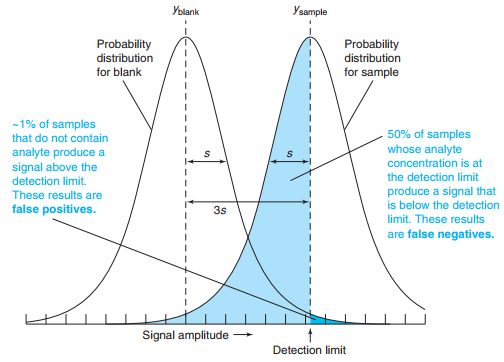
The detection limit is usually taken as 3 times the standard deviation of the blank.
The lower limit of quantitation is 10 times the standard deviation of the blank.
The reporting limit is the concentration below which regulations say that the analyte is reported as “not detected,” even when it is observed.
-
Robustness
Robustness is the ability of an analytical method to be unaffected by small, deliberate changes in operating parameters.
-
Standard Addition and Matrix Effect
In standard addition, known quantities of analyte are added to the unknown.
Standard addition is especially appropriate when the sample composition is unknown or complex and affects the analytical signal.
In such cases, it is impossible or difficult to create standards and blanks whose composition matches that of the sample.
If standards and blanks do not match the composition of the unknown sample, a calibration curve is not reliable.
The matrix is everything in the unknown, other than the analyte.
A matrix effect is a change in the analytical signal caused by anything in the sample other than the analyte.
-
An internal standard
An internal standard is a known amount of a compound different from an analyte that is added to the unknown.
The signal from the analyte is compared with the signal from the internal standard to find out how much analyte is present.
A carefully chosen internal standard will give an analytical signal (such as a chromatographic peak or spectrophotometric absorption) that is well separated from those of the analyte and other species in an unknown.
The internal standard should be chemically stable and not react with unknown components.
It is helpful for the internal standard to be chemically similar to the analyte so that uncontrolled effects of the matrix that increase or decrease the analyte signal might have a similar effect on the signal from the standard.
Internal standards are especially useful for analyses in which the quantity of sample analyzed or the instrument response varies slightly from run to run.
They are used in chromatography because the small quantity of sample injected into the chromatograph is not reproducible.
Internal standards are desirable when sample loss occurs during sample preparation steps before analysis.
If a known quantity of standard is added to the unknown before any manipulations, the ratio of standard to analyte remains constant because the same fraction of each is lost in any operation.
To use an internal standard, we prepare a known mixture of standard and analyte to measure the relative response of the detector to the two species.
However, the detector generally will have a different response to each component.
How to store/preserve Microorganisms
In summary;
Standard addition: The added standard is the same substance as the analyte.
Internal standard: The added standard is different from the analyte.
External standard: Solutions with known concentrations of analyte are used to prepare a calibration curve.
The matrix affects the magnitude of the analytical signal. In standard addition, all samples are in the same matrix.
Linear range: concentration range over which the calibration curve is linear
Dynamic range: concentration range over which there is measurable response.
Reproducibility: describes how well different people in different laboratories with different equipment can get the same results when analyzing similar samples by the same procedure.
Repeatability: describes how well one person can obtain the same results when analyzing the same sample by the same procedure with the same equipment in the same laboratory
Join the Enlighten Knowledge Community