Enlighten Knowledge Exam, Chemistry Revision Questions on Atomic Structure 2023 (P2)
1. Millikan performed an experiment method to determine which of the following?
A. Mass of the electron
B. Charge of the electron
C. e/m ratio of electron
D. Both (a) and (b)
2. Which is not true with respect to cathode rays?
A. A stream of electrons
B. Charged particles
C. Move with speed same as that of light
D. Can be deflected by magnetic fields
3.Which of the scientist were able to prove that atom is no longer non-divisible?
A. Dalton
B. Michael Faraday
C. Thomson
D. Chadwick.
4. Which of the following statements about the electron is incorrect?
A. It is negatively charged particle
B. The mass of electron is equal to the mass of neutron.
C. It is a basic constituent of all atoms.
D. It is a constituent of cathode rays.
5. When beryllium is bombarded with alpha particles (Chadwick’s experiment) extremely penetrating radiations, which cannot be deflected by electrical or magnetic field are given out. These are :
A. A beam of protons
B. Alpha rays
C. A beam of neutrons
D. A beam of neutrons and protons.
6.Which of the following sets of quantum numbers represents the highest energy of an atom?
A. n = 3, l = 0, m = 0, s = +1/2
B. n = 3, l = 1, m = 1, s = +1/2
C. n = 3, l = 2, m = 1, s = +1/2
D. n = 4, l = 0, m = 0, s = +1/2
7. What will be the sum of all possible values of l and m for n = 5 ?
A. 12
B. 4
C. 13
D.9
8. The orientation of an atomic orbital is governed by
A. Spin quantum number
B. Magnetic quantum number
C. Principal quantum number
D. Azimuthal quantum number
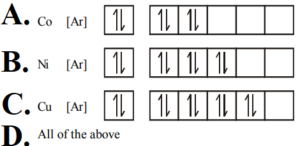
9. Which of the following is not correct for electronic distribution in the ground state?
10. Which of the following statement(s) is/are incorrect regarding photoelectric effect?
(i) The number of electrons ejected is proportional to the intensity of light.
(ii) There is some time lag between the striking of light beam on the metal surface and the ejection of electrons.
(iii) The kinetic energy of ejected electrons depends upon the brightness of light.
(iv) The kinetic energy of the ejected electron is proportional to the frequency of the incident radiation.
A. (i) and (ii)
B. (ii) and (iii)
C. (ii) only
D. (ii) and (iv)
11. Which of the following statements of quantum mechanics was in agreement with Bohr’s model?
i. The path of an electron in an atom can never be determined accurately.
ii. The energy of electrons in atom is quantized i.e., can only have specific values.
iii. An orbital cannot contain more than two electrons.
A. Only i
B. i and ii
C. Only ii
D. ii and iii
12. Which of the following statements concerning the quantum numbers are correct ?
i. Angular quantum number determines the three- dimensional shape of the orbital.
ii. The principal quantum number determines the orientation and energy of the orbital.
iii. Magnetic quantum number determines the size of the orbital.
iv. Spin quantum number of an electron determines the orientation of the spin of electron relative to the chosen axis.
A. i and ii
B. i and iv
C. iii and iv
D. ii, iii and iv
13. Arrange the following elements in the order of ease of detection of wave properties, in the de Broglie experiment. H, Li, Be, B, K
A. H < Be, B < Li < K.
B. H > Li > K > Be > B
C. H > Li > Be > B > K
D. H < Li < Be < B < K
14. An electron has principal quantum number 3. The number of its (i) subshells and (ii) orbitals would be respectively
A. 3 and 5
B. 3 and 7
C. 3 and 9
D. 2 and 5
15.

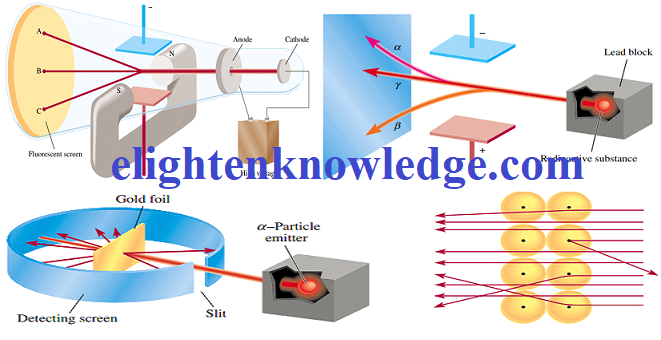
16. Give a brief description of the mass spectrometer
Answer:
A mass spectrometer consists of a vaporization chamber, an ionization chamber, an electric field, a magnetic field perpendicular to the electric field, and a detector.
17. State the functions of the main parts of the mass spectrometer
Answer:
Vaporization chamber:
The atoms are converted into vapor.
Ionization chamber:
The vaporized atoms are bombarded with a stream of high-energy electrons.
An electric field:
Accelerates the ions onto the magnetic field.
Magnetic field:
Deflects the ions into a semi-circular path.
Detector:
The ions give up their charges in the form of electric current.