Enlighten Knowledge Exam, Chemistry Revision Questions on Atomic Structure 2023 (P1)
1. According to Bohr’s theory, the angular momentum of an electron in the 5th orbit is…

2. In Bohr’s model, the atomic radius of the first orbit is y, and the radius of the 3rd orbit is
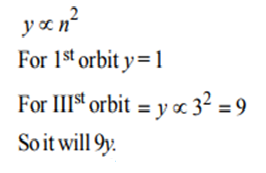
3. The radius of 1st Bohr’s orbit for the hydrogen atom is ‘r’. The radius of the second Bohr’s orbit is
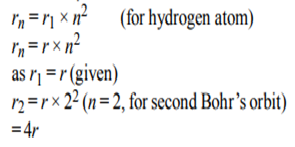
4. The energy of an electron in the nth Bohr orbit of a hydrogen atom is
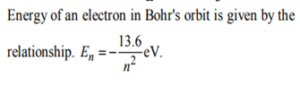
5. The energy of second the Bohr orbit of the hydrogen atom is 328 kJ/mol; hence the energy of the fourth Bohr orbit would be:
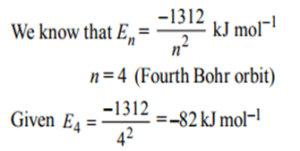
6. In a hydrogen atom, if the energy of an electron in the ground state is 13.6. eV, then that in the 2nd excited state is…
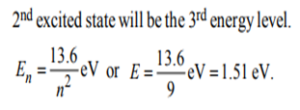
7. Which of the following transitions of electrons in the hydrogen atom will emit maximum energy?
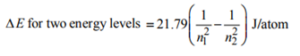
8. The energy of an electron in the second Bohr orbit of a hydrogen atom is…
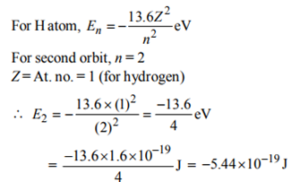
9. The ionization potential of a hydrogen atom is –13.6 eV. What will be the energy of the atom corresponding to n = 2?

10. The wavelength of the radiation emitted, when in a hydrogen atom electron falls from infinity to stationary state 1, would be (Rydberg constant = 1.097×107 m–1)
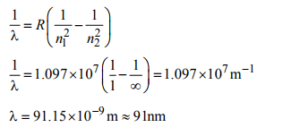
11. Which of the following statements do not form a part of Bohr’s model of the hydrogen atom?
A. Energy of the electrons in the orbits is quantized
B. The electron in the orbit nearest the nucleus has the lowest energy
C. Electrons revolve in different orbits around the nucleus
D. The position and velocity of the electrons in the orbit cannot be determined simultaneously.
12. Bohr model can explain :
A. the solar spectrum
B. the spectrum of the hydrogen molecule
C. spectrum of any atom or ion containing one electron only
D. the spectrum of hydrogen atoms only
13. Bohr’s model is not able to account for which of the following.
A. Stability of atom.
B. Spectrum of a neutral helium atom.
C. Energy of a free electron at rest.
D. Calculation of radii of the stationary states.
14. According to the Bohr theory, which of the following transitions in the hydrogen atom will give rise to the least energetic photon?
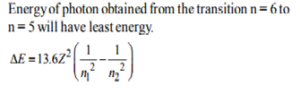
15. The wavelength (in cm) of the second line in the Lyman series of hydrogen atomic spectrum is (Rydberg constant = R/ cm)
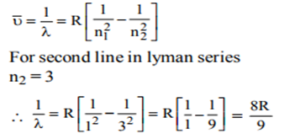
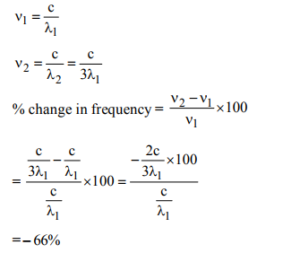
16. If the wavelength of the electromagnetic radiation is increased to thrice the digital value, then what will be the percent change in the value of frequency of the electromagnetic radiation?
17. Rutherford’s experiment on the scattering of -particles showed for the first time that the atom has :
A. electrons
B. nucleus
C. protons
D. neutrons
18. Rutherford’s -particle dispersion experiment concludes
A. all positive ions are deposited at a small part
B. all negative ions are deposited in a small part
C. proton moves around the electron
D. neutrons are charged particles.
19. Nucleons are
A. only neutrons
B. neutrons + protons
C. neutrons + protons + electrons
D. neutrons + electrons
20. Atoms with the same mass number but different atomic numbers are called
A. isotopes
B. isochores
C. isobars
D. None of these
21. When a metal surface is exposed to solar radiation
A. The emitted electrons have energy less than a maximum value of energy depending upon the frequency of incident radiations
B. The emitted electrons have energy less than the maximum value of energy depending upon the intensity of incident radiation
C. The emitted electrons have zero energy
D. The emitted electrons have energy equal to the energy of photons of incident light
22. The orbitals are called degenerate when
A. they have the same wave functions
B. they have the same wave functions but different energies
C. they have different wave functions but same energy
D. they have the same energy
23. The number of spherical nodes in 3p orbitals are
A. one
B. three
C. two
D. None of these
24. Number of unpaired electrons in N2+ is
A. 2
B. 0
C. 1
D. 3
25. In a given atom no two electrons can have the same values for all the four quantum numbers. This is called
A. Hund’s Rule
B. Aufbau principle
C. Uncertainty principle
D. Pauli’s exclusion principle
26. What can be the representation of the orbital having 3 angular nodes and n = 5.
A. 5d
B. 5f
C. 5p
D. 5s
27. Arrange the orbital of same shell in the increasing order of shielding strength of the outer shell of electrons.
s, f, d, p
A. s < p < d < f
B. s > p < d < f
C. s > p > d < f
D. s > p > d > f
28. If n = 6, the correct sequence for filling of electrons will be :
A. ns (n – 2) f (n – 1) d np
B. ns (n – 1) d (n – 2) f np
C. ns (n – 2) f np (n – 1) d
D. ns np (n – 1) d (n – 2) f
29. If the alpha-particles are projected against the following atoms Fe, Be, Mg, Al then increasing order in which the alpha-particle feel repulsion will be
A. Be, Mg, Al, Fe
B. Be, Al, Mg, Fe
C. Mg, Al, Mg, Fe
D. Al, Mg, Fe, Be
30. An electron has principal quantum number 3. The number of its (i) subshells and (ii) orbitals would be respectively.
A. 3 and 5
B. 3 and 9
C. 3 and 7
D. 2 and 5