How Pathogens are eliminated from the body and Why the Immune System Reject Foreign Tissues and Organs?

How Pathogens are eliminated from the body and Why the Immune System Reject Foreign Tissues and Organs?
Introduction
In combination with the leukocytes involved in the innate immune system, the cells of the adaptive immune system are almost always successful in eliminating threats from bacteria, parasites, fungi, and viruses.
The many different mechanisms used by the adaptive immune system to dispose of foreign invaders are broadly grouped into two responses:
- The cell-mediated response is promoted by TH1 cells and involves the activation of phagocytic cells and cytotoxic T lymphocytes (activated CD8+ cells), among others. This response primarily takes place through cell–cell contact.
- The humoral response is promoted by TH2 cells and involves the production of antibodies and other proteins secreted into the blood and lymph. (The Latin root humor means “fluid.”).
How Are Extracellular Pathogens Eliminated?
The innate immune response includes macrophages and dendritic cells that phagocytize some of the invaders at the site of infection.
In addition to killing the foreign cells, these leukocytes process and present antigens to the adaptive immune system via class II MHC proteins.
Macrophages display the processed epitopes on their surfaces at the site of infection, while dendritic cells do so in the lymph nodes.
If the epitopes are recognized by helper T cells, the now-activated T cells move into the site of infection.
If an activated TH1 cell binds to the class II MHC proteins presented by antigen-laden macrophages, two things happen.
First, the phagocytic activity of the macrophages is enhanced.
Second, the TH1 cells secrete cytokines that recruit additional phagocytic cells to the site increasing the inflammatory response.
In addition, antibodies from plasma cells begin attaching to extracellular bacteria, fungi, viruses, and other foreign material.
These bound antibodies interfere with the infection in four ways.
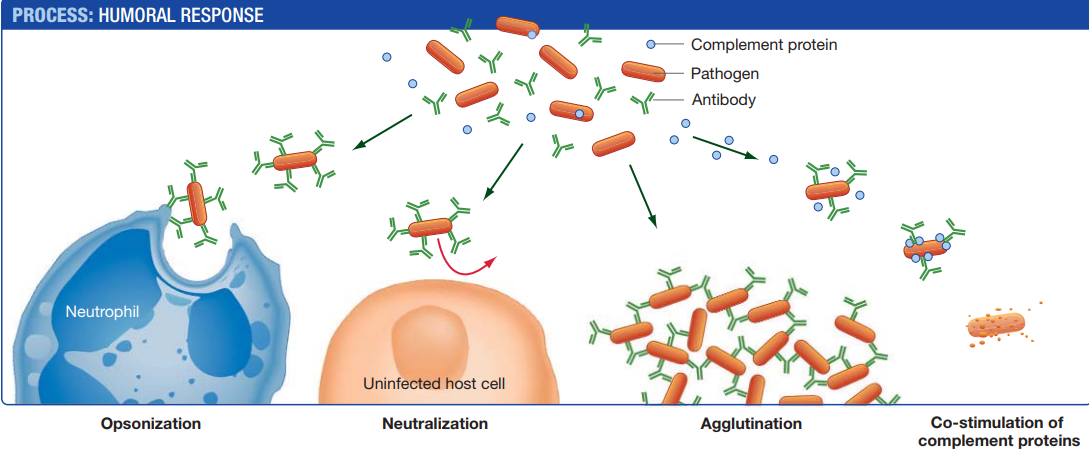
1. Opsonization (“preparation for eating”)
- Antibodies from plasma cells coat pathogens at the infection site.
- Pathogens that are coated with antibodies are readily destroyed by phagocytes.
2. Neutralization
- Coated pathogens are blocked from interacting with and thus infecting host cells.
- Their participation in the infection is neutralized.
3. Agglutination (“gluing together”)
- In many cases, antibodies cause the clumping of antigens, including cells and viruses, through a process called agglutination.
- Each antibody has at least two binding sites, so a single antibody can bind epitopes on cells or viruses and cross-link them.
- Clumped cells and viruses cannot infect the cells of the body and are easy targets for phagocytes.
4. Co-stimulation of complement proteins
- Antibodies that are bound to pathogens also activate a lethal group of proteins called the complement system.
- Complement proteins circulate in the bloodstream and assemble at antigen–antibody complexes.
- When complement proteins are activated, they punch deadly holes in the plasma membranes of pathogens.
- Within a few days, this combination of killing mechanisms, armies of phagocytic cells, and complement proteins that home in on antibody-tagged material, usually eliminates all of the extracellular pathogens.
But what about those pathogens that reside within the cells of the body?
How Intracellular Pathogens Eliminated
Most nucleated cells in the body express class I MHC proteins on their cell surface.
Recall that these MHC proteins display peptides that were processed from cytosolic proteins.
This means that if a cell were infected, peptides from the foreign proteins present inside the cell would be loaded onto some of the MHC proteins and presented to circulating T cells.
Cells that display the antigens of intracellular pathogens are effectively waving a flag that says,
- “I’m infected.
- If you destroy me,
- You’ll destroy the infection.”
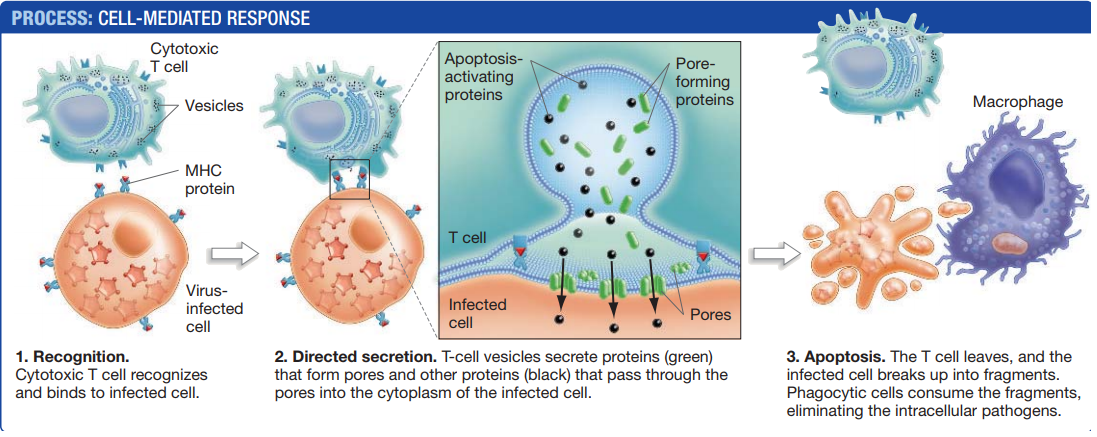
As cytotoxic T cells migrate into the area, those that recognize the class I MHC–peptide complex are stimulated to respond to the signal.
After cytotoxic T cells recognize their target, they form a tight attachment with the cell that directs the secretion of molecules from the T cell to the target cell’s surface, without exposing adjacent cells to those molecules.
Some of the molecules are proteins that assemble into pores in the target cell’s plasma membrane.
These pores allow other proteins from the T cell to pass directly into the cytoplasm of the target cell, where they activate a signalling cascade that causes the cell to self-destruct through apoptosis.
The result of apoptosis is the death and fragmentation of a cell into smaller vesicles, called apoptotic bodies, which are ingested by phagocytes like macrophages.
Once the cytotoxic T cell has delivered this signal, often referred to as the “kiss of death,” the T cell releases the dying cell and seeks out another infected cell to kill.
Over time, all of the infected cells are eliminated.
If the pathogen can replicate only inside host cells, as is the case with viruses, this cell-mediated response limits the spread of the infection by preventing the production of new generations of pathogens.
Why Does the Immune System Reject Foreign Tissues and Organs?
- The innate and adaptive immune responses have devastating effects on invading pathogens.
- Unfortunately, they are equally deadly in their responses to tissues or organs that are introduced into a patient to heal a wound or cure a disease.
- Consider the problems that can arise with blood transfusions.
- You might recall that certain individuals have red blood cells with membrane glycoproteins called A and B.
- These molecules act as antigens if they are introduced into a per- son whose own blood cells lack those glycoproteins.
For example,
If you have type A blood, it means that your blood cells have the A glycoprotein.
If your blood is transfused into a person who lacks the A glycoprotein meaning someone who has type B or type O blood the recipient’s immune system will recognize the A glycoprotein as an antigen and mount a response against it.
For a blood transfusion to be successful, the recipient must be given blood that contains the same glycoproteins found on his or her blood cells or that lacks the A and B glycoproteins entirely (type O).
Similar problems arise in organ transplants, except that the molecules directing the rejection of these tissues are the class I MHC proteins.
The adaptive immune system mounts a response against the foreign MHC proteins, and the innate response is activated based on the absence of “self MHC” signals.
If transplanted cells do not display the same class I MHC proteins as host cells, components of the innate immune system kill them.
To prevent strong immune reactions against transplanted organs or tissues, physicians seek donors who have MHC proteins that are extremely similar to those of the recipient, as is often the case for siblings.
Close relatives, however, molecular differences will exist between the donor and recipient.
Thus, physicians must also treat the recipient with drugs that suppress the immune response.
Thanks to steady improvements in drug development and in systems for matching MHC types between donors and recipients, the success rate for organ transplants has improved dramatically in recent years.
As the blood transfusion and organ transplant examples show, the immune system rejects foreign cells because they either contain nonself molecules or lack self-molecules.
To your immune system, a mismatched blood transfusion or an organ transplant is indistinguishable from a massive influx of bacteria, viruses, or other foreign invaders.
Join Enlighten Knowledge WhatsApp platform.
Join Enlighten Knowledge Telegram platform.
Follow our:
FACEBOOK PAGE.